Abstract
Introduction
Immunomodulatory/cereblon-binding drugs (IMiDs), including lenalidomide and pomalidomide, have improved survival of patients with multiple myeloma (MM) and comprise the therapeutic foundation at all phases of therapy. While these agents are generally well-tolerated, their increased risk of venous thromboembolism (VTE), in particular deep vein thrombosis and pulmonary embolism, presents a major clinical challenge for the treatment of MM. Apixaban, a direct oral anticoagulant which directly blocks Factor Xa, has been approved for treatment in patients with VTE. Apixaban has not been prospectively evaluated for thromboprophylaxis in MM in the US. In this phase IV single-arm study (NCT02958969), we prospectively evaluate the safety and efficacy of apixaban for primary prevention of VTE in patients with MM receiving IMiD therapy.
Methods
Fifty patients with MM on IMiD therapy received apixaban 2.5 mg orally twice daily for primary prevention of VTE and were prospectively monitored for 6 months. Patients requiring therapeutic anticoagulation or with history of prior VTE were excluded. Patients stopped aspirin while on apixaban. Primary safety outcomes were rates of major hemorrhage and clinically relevant non-major hemorrhage over 6 months. Major bleeding was defined as overt bleeding associated with a decrease in hemoglobin of ≥ 2 g/dL, requiring transfusion of ≥ 2 units of blood, occurring in a critical site, or contributing to death. Clinically relevant non-major bleeding was defined as overt bleeding not meeting the criteria for major bleeding but associated with medical intervention, surgical intervention, or interruption of the study drug. The primary efficacy outcome was the rate of symptomatic VTE over 6 months.
Results
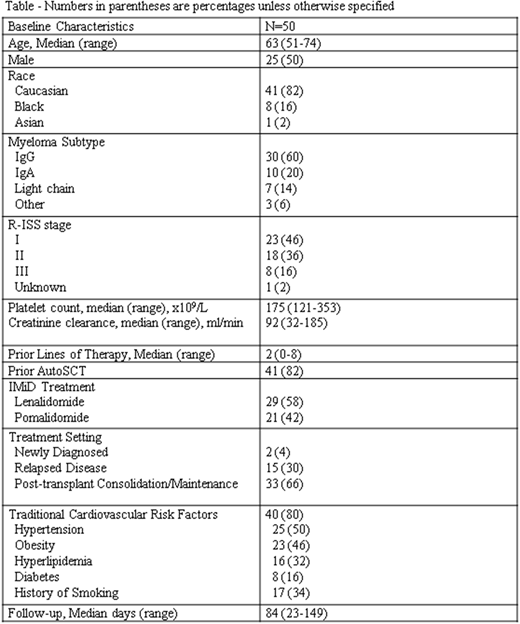
Baseline characteristics are listed (Table). Median age was 63 years (range 51-74) with 50% of patients being male. Patients had received a median of 2 lines of therapy (range 0-8), and the majority (82%) had received prior autologous stem cell transplantation (AutoSCT). Patients received apixaban thromboprophylaxis in combination with lenalidomide (58%) or pomalidomide (42%). The majority (66%) received IMiDs for post-autoSCT consolidation or maintenance therapy. Most (80%) had traditional cardiovascular (CV) risk factors prior to initiation of apixaban, including hypertension (50%), obesity (46%), history of smoking (34%) and hyperlipidemia (32%).
At planned interim analysis at 3 months (range 23-149 days) with data still being collected for planned 6 month study duration, no patients had experienced major hemorrhage or VTE. Two patients experienced clinically relevant, non-major hemorrhage, including one patient with unprovoked epistaxis lasting more than 5 minutes and another patient with mechanical trauma. These events were medically managed, and both patients were able to resume apixaban. One patient stopped therapy shortly after initiation due to allergic reaction to apixaban manifesting as generalized edema. No patients experienced stroke, myocardial infarction (MI), or death.
Conclusions
In this pilot study of 50 patients, low-dose apixaban was safe and well tolerated as thromboprophylaxis for patients with MM receiving IMiDs. No patients experienced VTE, major hemorrhage, stroke, or MI. Further randomized studies are needed to validate apixaban as a standard primary prevention anti-thrombotic strategy for patients with MM receiving IMiDs.
Moslehi:Bristol-Myers Squibb: Consultancy, Research Funding. Jagasia:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal